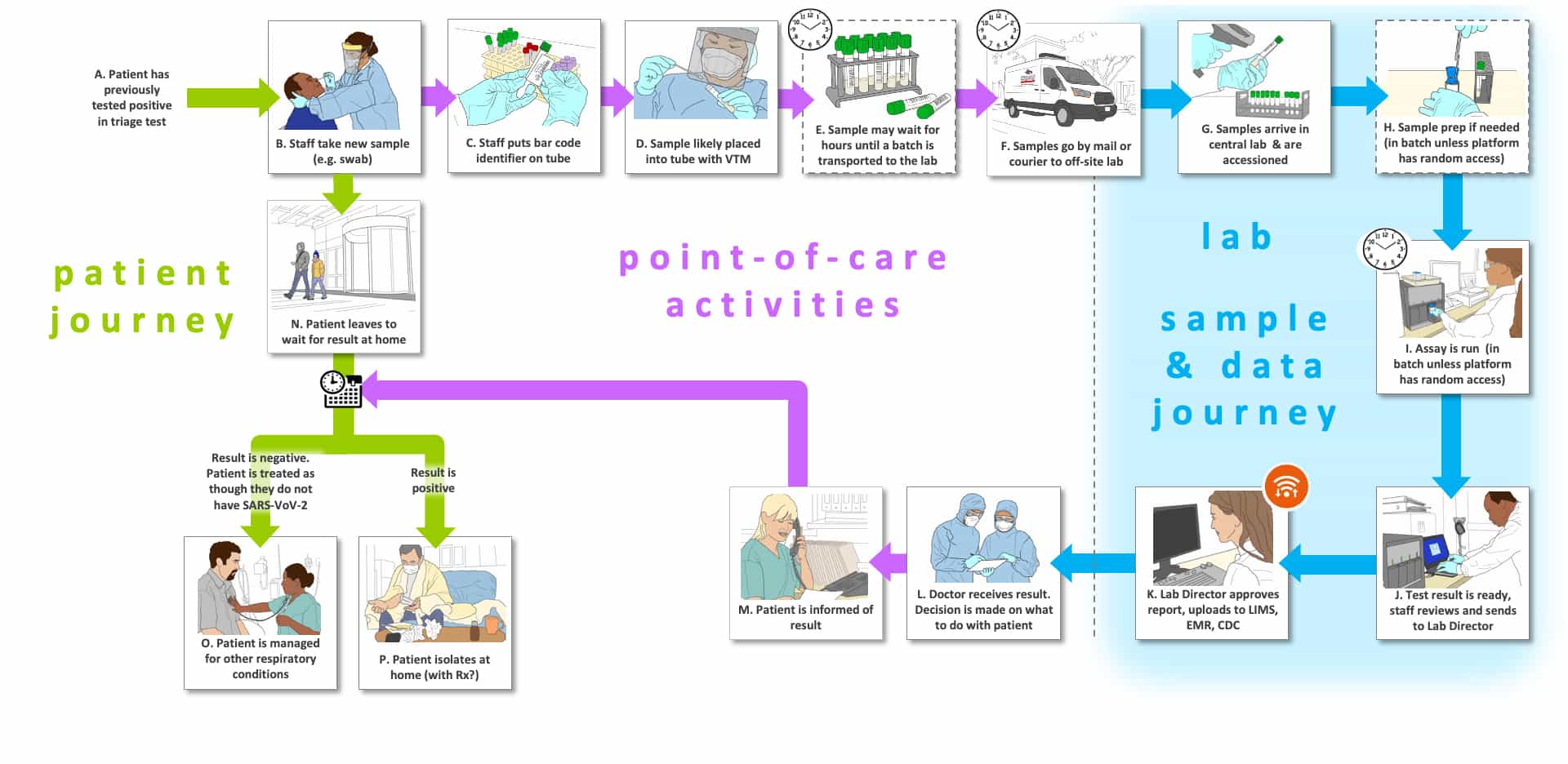
Storyboard B, which aligns with our Use Case 4, begins from O1 in Storyboard A, where the patient must wait for the results of an offsite confirmation test result. The sample is most likely taken at the same site as the triage test (Step B) and the patient goes home to await results, which interrupts the patient pathway until the confirmation results are ready. In the sample pathway within the point of care environment, the healthcare worker now prepares the sample for shipment to a lab offsite (Steps C-F). It is possible that the samples will wait for a substantial time (Step E) until there are sufficient samples to justify shipment, especially if there is a minimum batch required by the partner lab that will provide confirmation testing. The samples could be sent by courier to a local offsite lab (Step F) or shipped in some fashion (not shown).
The lab sample and data journey in Steps G-K are similar to Storyboard A, except that we now assume that the system is in a moderately complex or central lab that will perform the confirmation tests in batch. We also assume that today the test will be an RNA assay, which could be PCR, isothermal amplification or NGS. Conceivably, if the performance is shown to be sufficient, an antigen test could be employed. Several are available or under development.
Since the patient is no longer on-site (Step N), once the clinician has the result of the test (L), there will be a mechanism to communicate with the patient (Step M). If the test confirms SARS-CoV-2 infection, the patient will receive self-care instructions while isolated at home and provided warning signs that would suggest that seeking further elevated healthcare would be necessary. If the test is negative, the patient will be instructed to seek appropriate care from their primary care provider.
Although the use of a send-out confirmation is less attractive than on-site testing, the benefits of triage and confirmation testing to improve the efficiency of our RNA testing capacity could easily justify the off-site confirmation testing setting as well. The best reason for this is to decrease the number of RNA tests ordered that will almost certainly involve a large number of true negatives and therefore unnecessary and costly.
The Storyboards are meant to be approximate descriptions of the overall testing ecosystem. They are organized as flow charts containing sites of activity, people involved (e.g., patients, medical practitioners and laboratorians) and pathways for tested individuals, healthcare professionals (or other testers in some cases), sample collection and transport, testing, result generation and information flow. They also show key decisions informed by the test results.
There are three types of “journeys” in the Storyboards: 1) the tested individual’s (usually patient) journey which are shown using green arrows, 2) point-of-care or point-of-use activities which are shown with purple arrows (e.g., sample collection, sometimes testing) and 3) the sample and data journey through a laboratory, which is shown with blue arrows.
The letters that label each step are not meant to indicate an order for the steps, they are simply there to facilitate discussion about the storyboard. Optional steps have a dashed outline, and examples of possible variations in a step are labeled with the same letter followed by differentiating numbers e.g., B1, B2 and B3)
There are a number of clocks and calendars pictured near specific steps to indicate time-consuming steps and those that could vary in total time depending upon the workflow efficiency of the healthcare site and the characteristics of the testing platform (e.g., batch analysis, time to results).