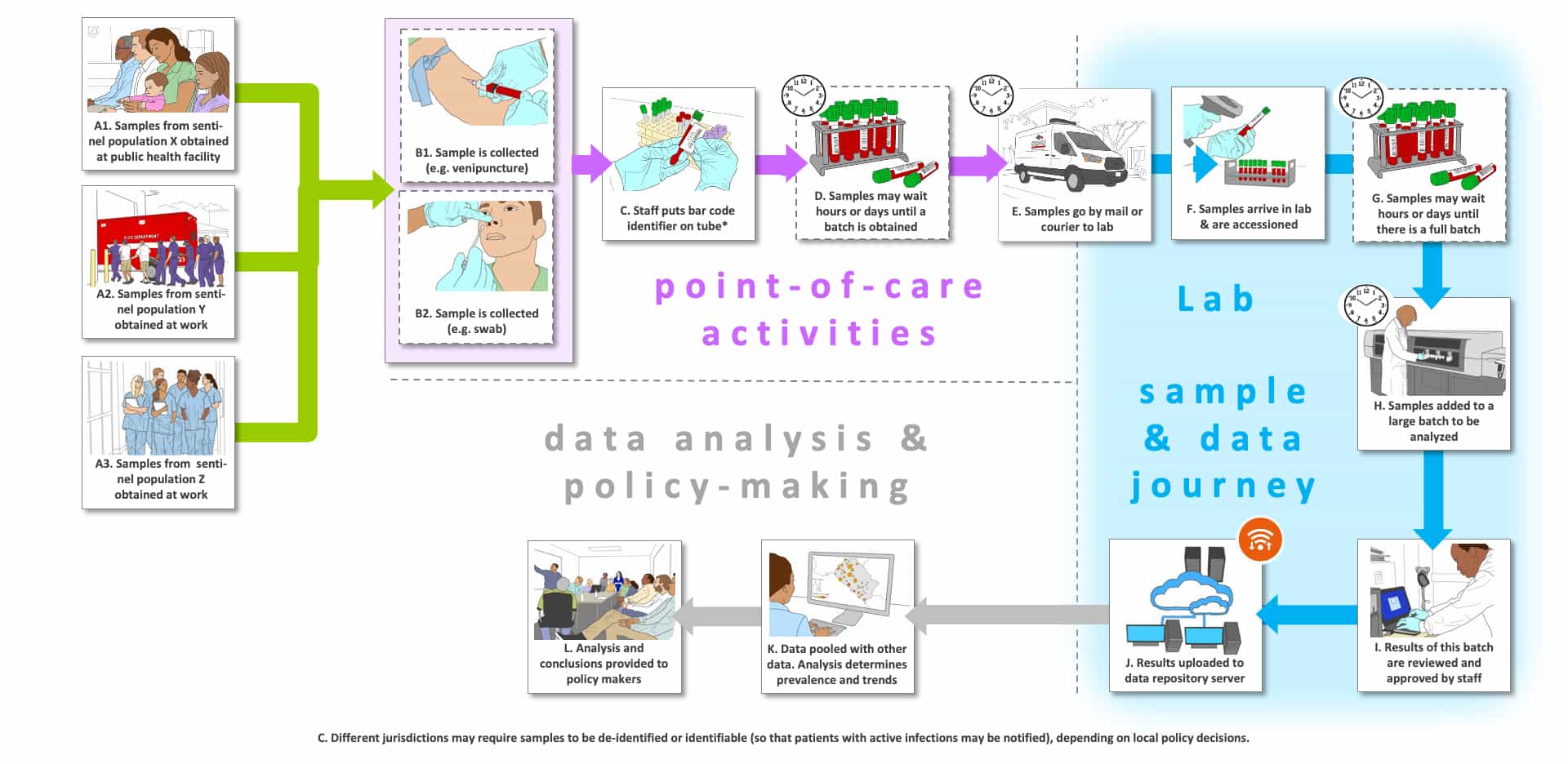
Storyboard G is envisioned for Use Case 8, which has been modified from our previous version that was first published in April 2020. Use Case 8 now illustrates three intended uses for surveillance of a sentinel population: to determine 1) an estimation of incidence of current SARS-CoV-2 infection in symptomatic persons (our lone previous intended use), 2) an estimation of incidence of current SARS-CoV-2 infection in non-symptomatic persons (new addition) or 3) an estimation of the prevalence of SARS-CoV-2 infection based upon prior exposure to SARS-CoV-2 test (new addition). The corrected Use Case 8 has these new definitions.
The Storyboard describes population testing, not individual testing. We believe that a diagnostic test (RNA or antigen; Use Case 5) will be used for intended use 1 and 2, while a test of previous exposure (IgG alone or potentially in combination with IgM and/or IgA; Use Case 7) can be used for intended use 3. If the intention of the survey is to determine SARS-CoV-2 incidence as well as other respiratory pathogens, the differential diagnostic test (Use Case 6) could be used for intended uses 1 and 2.
In Storyboard G, we note that different localities could select different sentinel populations (Steps A1, A2 and A3). Step A1 depicts intended use 1 for symptomatic individuals. In an endemic setting we suppose that a local public health facility could be the site of collection. We have not shown extensive PPE in use, although this will depend upon local conditions (low or high prevalence). In Step H, often large batches of samples are analyzed together to improve cost-per-sample. It is conceivable that this setting could be used for intended use 3 as well, in which case blood would likely be drawn (venipuncture or finger prick), although immunoassays that employ respiratory or oral samples have been developed. Steps A2 and A3 could involve sentinel populations (e.g., first responders, healthcare workers) for either intended use 1 or 2 (symptomatic, asymptomatic or pre-symptomatic). We assumed that higher-risk populations would be preferred so that statistically meaningful sample sizes could be tested without requiring very large numbers of subjects. Note that in contrast to the previous Storyboards, the patient journey ends after the sample is taken.
We assume that the most likely scenario is off-site testing. The completion of surveillance for a population is not likely to be particularly time-sensitive. In Steps D and E, samples may wait for hours or longer until large batches are accumulated. In some locations, samples may wait at room temperature. Sample transport could also take significant time to reach large non-regional labs with the capacity to process a large number (100’s to 1,000’s) of samples. Surveillance testing is often a very cost-sensitive market. As shown in Step G, often large batches of samples are analyzed together to improve cost-per-sample. Platforms with low costs-per-sample will have an advantage (Step H).
We envisioned in this storyboard that sample results would not be returned to a physician or patient, but instead would be sent to specialized epidemiology groups for analysis (e.g., bioinformatics “middle-ware” solutions groups) and then to the appropriate public health officials and policy makers. The grey “data analysis and policy making” track (Steps K and L) is found only in this Storyboard. Note that data may be identified or de-identified depending upon location and policies in place. We have not depicted the “test and treat” option mentioned below, which has sometimes been used in surveillance studies for other infectious diseases.
We believe that surveillance testing will become quite common. Several studies have already been reported for intended use 2 and 3, but to our knowledge these processes have not been adopted as routine ongoing processes. Initially, the current infection rate and previous exposure will be determined in selected sentinel populations. As time goes on there will likely be updated analyses in the same sentinel populations to determine a number of metrics. Are the number of current infections changing (change in incidence)? This will be an indirect measure of overall intervention program success. Is the prevalence changing? It is likely that the immune status will be cumulative within the population, so the rate of change would need to be measured over time. In both cases, the statistical power of the studies will depend upon the representation of the sentinel populations, the size of the study, the size of the change required to be detectable, and the performance of the tests employed. In addition, if the immunoglobulin tests can be correlated to protective immunity, it should be possible to monitor the titer (and possibly the epitope specificity) of immunoglobulins in the samples as a measure of changes in the immune status of the population. Is the titer or affinity decreasing? Over what period of time? This will be particularly important after vaccines are available, but then we will need tests that detect immunity independent of whether a person is or is not vaccinated. One other potential complication is that if the titer is decreasing to below detection in one set of subjects while the number of cases increases, it could appear as though prevalence is decreasing, but it is not. In this case it would be necessary to know the change in titer on a per-person basis and to differentiate old and new cases.
The individual results from the surveillance studies may or may not be returned to individual patients or their healthcare providers. This will vary based upon jurisdiction, intent of testing and/or local public health policies. In our experience with neglected tropical disease surveillance programs for schistosomiasis, soil transmitted helminths and onchocerciasis, it is common for researchers to supply surveillance test results to local healthcare professionals in order to provide a “test and treat” option for any new infections that are found during the course of a surveillance program. In an epidemic, this should be essential for tests that detect current infections, but not for tests that detect prior exposure. This is ancillary to the surveillance data collection.
The Storyboards are meant to be approximate descriptions of the overall testing ecosystem. They are organized as flow charts containing sites of activity, people involved (e.g., patients, medical practitioners and laboratorians) and pathways for tested individuals, healthcare professionals (or other testers in some cases), sample collection and transport, testing, result generation and information flow. They also show key decisions informed by the test results.
There are four types of “journeys” shown in this Storyboard: 1) the tested individual’s (usually patient) journey which are shown using green arrows, 2) point-of-care or point-of-use activities which are shown with purple arrows (e.g., sample collection, sometimes testing), 3) the sample and data journey through a laboratory, which is shown with blue arrows, and 4) the use of the data for further analysis and policy-making, which is shown with grey arrows.
The letters that label each step are not meant to indicate an order for the steps, they are simply there to facilitate discussion about the storyboard. Optional steps have a dashed outline, and examples of possible variations in a step are labeled with the same letter followed by differentiating numbers e.g., B1, B2 and B3)
There are a number of clocks and calendars pictured near specific steps to indicate time-consuming steps and those that could vary in total time depending upon the workflow efficiency of the healthcare site and the characteristics of the testing platform (e.g., batch analysis, time to results).