Portfolio Planning
Companies wishing to compete successfully in clinical diagnostics must compare and balance the technical difficulty of developing, delivering and supporting a new set of clinical diagnostic products with the potential financial return offered by those products.
Challenge
Succeeding in the clinical diagnostics business requires the focused application of resources to the development of a commercially viable portfolio of related products and services.
Once a clear understanding is gained regarding how the company’s technology might be applied to address unmet medical and market needs, the company must determine which opportunities are the best lead candidates for a new assay panel and which are best suited to broaden a panel offering. Since “one-product companies” are rarely successful, it is also important to understand what portfolio of products will best serve medical and market needs while leveraging the unique features of the company’s technology.
Strategic Approach
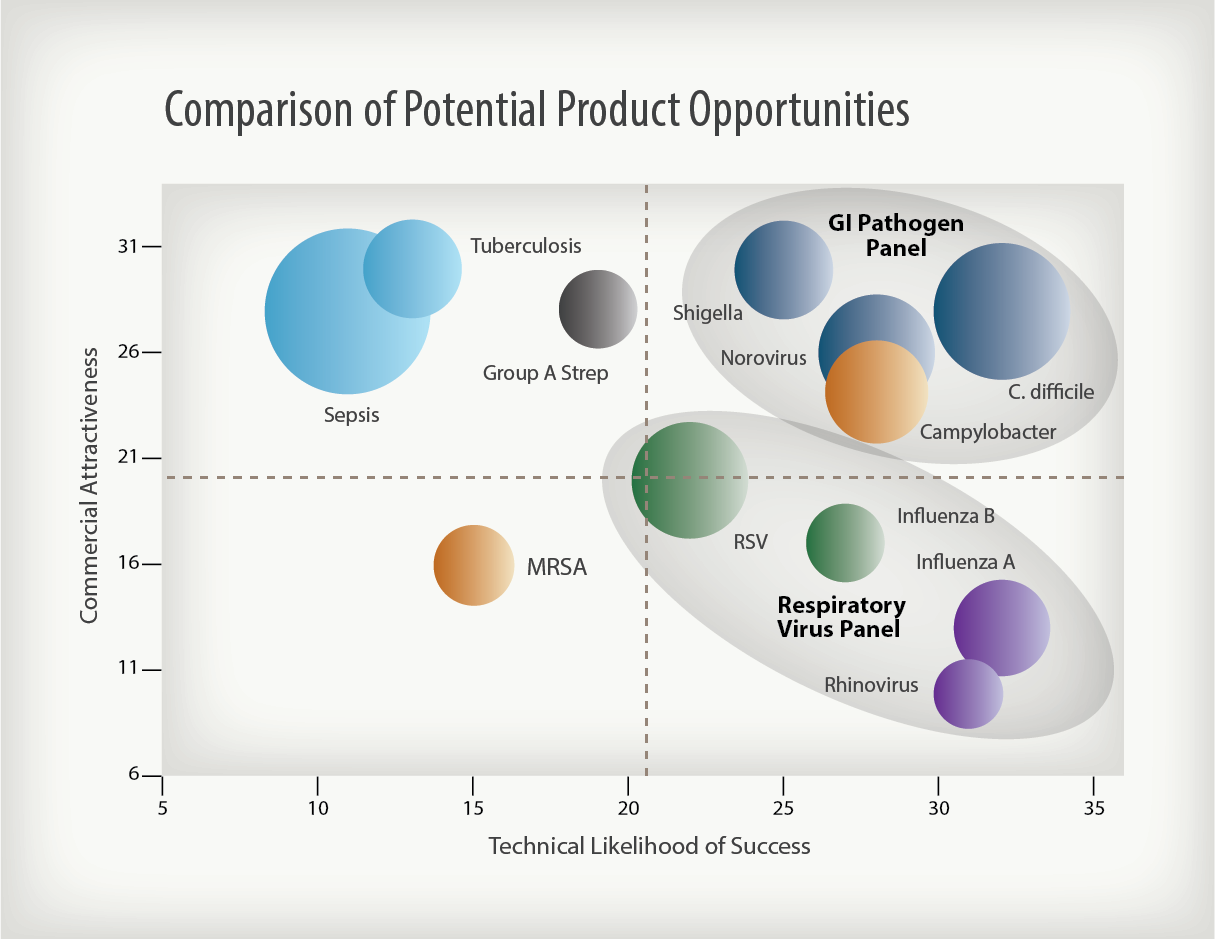
Portfolio planning is an essential requirement for success. One approach is to conduct an Opportunity Assessment to evaluate potential product opportunities from two perspectives: Commercial Attractiveness (CA) and Technical Likelihood of Success (TLS).
Conducting an Opportunity Assessment begins with evaluating the commercial potential of the company’s initial products to determine which might generate the most attractive revenue streams to fund future development, create value and satisfy investors. This is the CA Assessment. The other half of this exercise considers the technical difficulty of delivering a new product to market. If significant technical barriers must be overcome in order to commercialize the product, the revenue stream will be delayed and company value will build more slowly. This is the TLS Assessment. The “CA/TLS process” is a systematic, objective approach for ranking product opportunities in order to ultimately focus on the most attractive applications when developing the company’s lead products. Some opportunities, although not attractive as stand-alone products, might add incremental value to a portfolio of products and, thus, might be successfully added to the overall product development program. Such an analysis will help the company determine where to focus its research, development, business and commercial resources in order to maximize value and address the appropriate medical and market needs.
Result
Halteres Associates utilizes a proprietary, systematic process to assist its clients with assessing their commercial opportunities and objectively evaluating the technical challenges they face in delivering competitive product solutions.
HALTERES' CORE COMPETENCIES
Services
- Business and Financial Modeling
- Business Development
- Clinical Studies and Regulatory
- Global Sales and Marketing
- Impact Modeling and Value Creation
- Intellectual Property
- Investor Presentations
- Market and Technology Assessments
- Medical Practice
- Portfolio Planning
- Product and Process Development
- Strategic and Tactical Planning
Technologies
- Algorithms / Informatics / Health IT
- Biomarker Discovery and Validation
- Biostatistics
- Companion Diagnostics
- Digital Health and Wellness
- Histology / Anatomic and Clinical Pathology
- Immunodiagnostics
- Instrument Systems
- Life Science Research Tools
- Medical Devices
- Molecular Diagnostics
- Next Generation Sequencing
Markets
- Blood Screening
- Chronic Diseases
- Clinical Laboratory Services
- Global Health
- Infectious and Chronic Diseases
- Life Science Research
- Oncology
- “Omics” applications
- Parasitology
- Pathology
- Point of Care Testing
