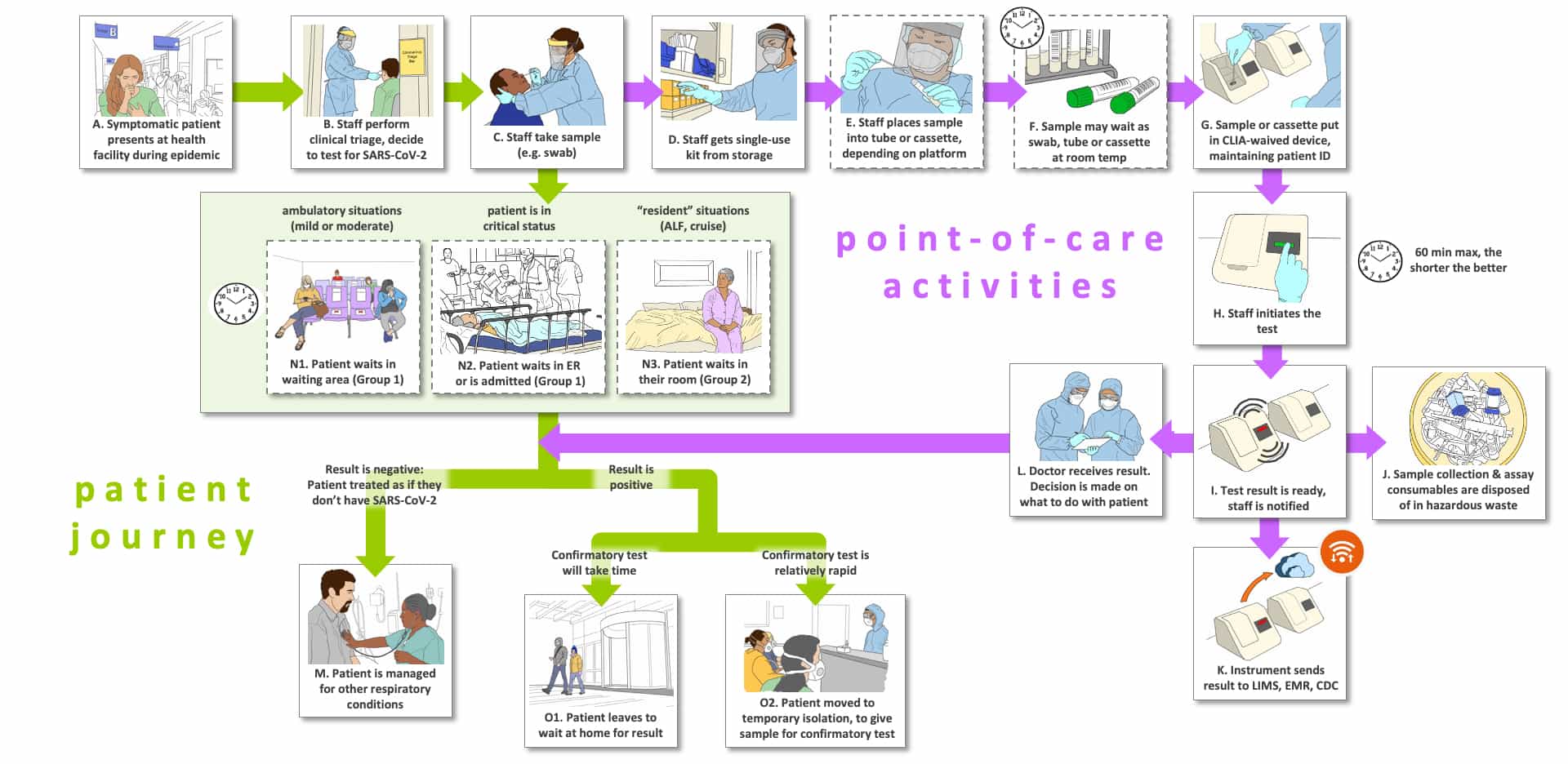
Storyboard A represents a triage setting described in our Use Cases 1 and 2, where a triage test is performed to capture most of the positive samples with a simple, inexpensive, fast test, typically to be followed by subsequent confirmation with a diagnostic test. The ideal triage test would have a high sensitivity so as not to miss positive individuals (see Use Case tables). Since the next step will be to conduct a confirmatory test, there will be an acceptable number of false positive results. But the number of acceptable false positives will be dependent upon the setting, whether PPE is required and the timing for the confirmatory results.
In Step A, a person presents with symptoms that they fear indicate they have COVID-19. A clinical assessment is performed and the medical practitioner decides the person should be tested for possible SARS-CoV-2 infection (Step B). The person is sent to a site for sample collection where a healthcare worker performs a sample collection (Step C). Note that the journey of the person tested and their sample now move in different flows.
For the patient journey, there are three main options depending upon where the person to be tested enters the system (Steps N1-N3). Steps N1 and N2 constitute the Group 1 sites (temporary isolation) we described in Use Case 1, while Panel N3 represents Group 2 (residential isolation). If the tested persons are in a waiting room with sufficient distancing and PPE (Step N1) or in an emergency room without symptoms sufficient to warrant immediate admission (N2), they are likely to wait for the test results then move to Steps M and O, that we will return to once the results are available. In contrast, persons living in an assisted living facility (N3) will likely remain in their room until results are available. Now we return to Step C to follow the sample.
In the sample journey, the healthcare professional that collected the sample obtains a test cartridge (Step D) and transfers the sample to the cartridge (Step E). Step F indicates that samples might sit for some time based on the lab workflow processes or that the system used is a batch analyzer. Because the test must be very fast, in most cases we believe that a single-bay system will work best for the triage setting, dependent upon the number of persons presenting with symptoms. For this reason, we show Step F as optional (dotted lines).
Steps G-L indicate cartridge loading and processes specific to the platform. The sample cartridge is placed into the instrument system while maintaining patient identity (G), the testing is initiated (H), the test is completed and the instrument notifies the appropriate staff member that test is complete (I). Reporting can be in a myriad of types, from paper to images on a screen to direct storage on a server. The cartridge is properly disposed of or potentially (hopefully) recycled (J). We assume that a properly designed system will have the appropriate connectivity and communication capability to deliver data concerning test results, instrument performance, patient ID and other information to the appropriate stakeholders (K). Now the sample journey is complete.
The patient and the test results now meet through the healthcare worker who will deliver the results to the patient (purple arrow meets the green arrows beneath Steps N1-N3). There are three paths shown for the patient based upon the test results. In Step M, the triage test results are found to be negative. Since a triage test needs a high negative predictive value to be of use, the individual is told they probably do not have COVID-19 and are sent home or managed for their symptoms. In Step O1, it is assumed that the confirmation test will take too long to wait for the results probably because the patient sample will be sent off-site for testing (or the patient doesn’t wish to wait for a result). This setting is taken up in Storyboard B (corresponding to Use Case 4). In O2, the confirmation test will be available quickly, almost certainly because the test can be performed at the same site as the triage test. We do not show another sample collection for the on-site confirmation testing setting, but it would be necessary unless two samples were taken at the first presentation of the patient with symptoms (which we believe is unlikely to occur). For the situation where the confirmation test will be conducted off-site, please see Storyboard B.
We have only shown nasopharyngeal swabs for sample collection, although we recognize there is a major push to move to alternate samples such as mid-turbinate swabs and saliva. Also, home collection of samples and shipment would be possible, but we believe that is far more useful in diagnosis and screening as opposed to the two-step triage and confirmation testing scenario.
If the confirmatory test can be conducted quickly (within hours) with temporary isolation of the patient at the clinic or at home, a specificity of as little as 50% could be acceptable. However, if there is a need to send the triage positive individual’s sample to an off-site lab, which currently can take 2-13 days for a result, there will be many people waiting in isolation needlessly for days. This is less of an issue for Group 2 sites where people are already in extended isolation since they are in residence. In the event that an individual has checked into an emergency room they are likely to have severe symptoms in which case they will either need a rapid triage and confirmation or just a diagnostic test. In this case, triage testing is quite unlikely to be useful with off-site confirmation testing. At this point we have not modeled the impact and the acceptable performance in these settings.
Note that to our knowledge there are no triage tests available today, but we are aware of multiple tests under development that could serve as triage tests that would use finger-prick blood, breath collection or saliva in rapid, inexpensive test formats. We do not consider RNA tests to be reasonable candidates for triage testing because they often take too long, are too expensive and/or have performance that is sufficient to serve as a true diagnostic test. However, RNA tests are the most likely confirmation tests for the foreseeable future. It is conceivable that effective clinical evaluation and questionnaires could approach the effectiveness of a lab test to triage persons presenting for testing.
There is a potential value to triage and confirmation testing that is not immediately obvious. Most RNA tests in persons with flu-like symptoms are negative. That is, those persons are probably not infected with SARS-CoV-2. As a result, the majority of the RNA tests are actually wasted. But, without some form of enrichment of populations to be tested by some means, we can’t improve RNA test utilization. If the triage approach is used and can be scaled, the number of RNA tests needed will decrease substantially. With a triage test with 99% sensitivity (very few false negatives) and 50% specificity and an environment where only 10% of the RNA tests are positive without triage testing, using a triage test before ordering an RNA confirmation/diagnosis test would save 80% of the RNA testing used today. That is, the effectiveness of RNA testing would increase 8-fold without adding any additional RNA test capacity.
The Storyboards are meant to be approximate descriptions of the overall testing ecosystem. They are organized as flow charts containing sites of activity, people involved (e.g., patients, medical practitioners and laboratorians) and pathways for tested individuals, healthcare professionals (or other testers in some cases), sample collection and transport, testing, result generation and information flow. They also show key decisions informed by the test results.
There are three types of “journeys” in the Storyboards: 1) the tested individual’s (usually patient) journey which are shown using green arrows, 2) point-of-care or point-of-use activities which are shown with purple arrows (e.g., sample collection, sometimes testing) and 3) the sample and data journey through a laboratory, which is shown with blue arrows.
The letters that label each step are not meant to indicate an order for the steps, they are simply there to facilitate discussion about the storyboard. Optional steps have a dashed outline, and examples of possible variations in a step are labeled with the same letter followed by differentiating numbers e.g., B1, B2 and B3)
There are a number of clocks and calendars pictured near specific steps to indicate time-consuming steps and those that could vary in total time depending upon the workflow efficiency of the healthcare site and the characteristics of the testing platform (e.g., batch analysis, time to results).