We have envisioned two Use Settings (storyboards) for our Use Case 5: one setting for the point-of-care diagnostic setting (on-site testing; Storyboard C) and one setting for the send-out to an off-site lab (Storyboard D). We also have depicted the point-of-care setting in an epidemic setting in Storyboard C, while we use an endemic setting for Storyboard D. Although Use Case 5 covers both the epidemic and endemic settings, we wanted to contrast the settings and their implications.
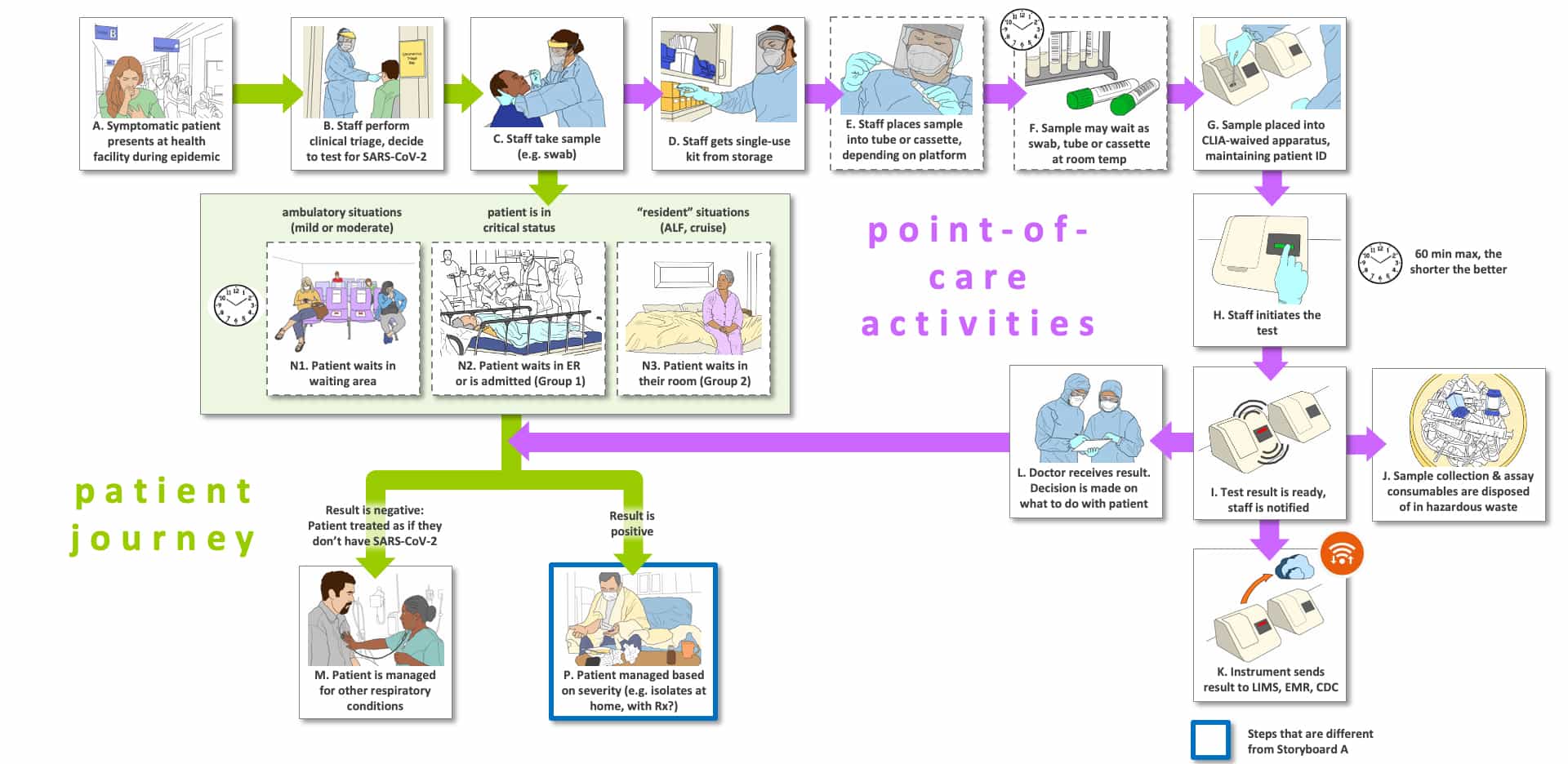
Note that Storyboard C is very much like Storyboard A. For the diagnostics scenario to function well, the test employed must have substantially better performance than the triage test we described. Both the triage test and the diagnostic test must have high sensitivity, but the diagnostic test is differentiated by very high specificity as well. The diagnostics tests today are typically SARS-CoV-2 RNA-based, take longer to perform than an acceptable triage test and cost 5-10-fold more than our envisioned triage test. The major change in Storyboard C is that Steps O1 and O2 from Storyboard A are replaced by Step P. Now there is no confirmation testing required, a definitive diagnosis is obtained via only one test, and the tested person with a positive result is assumed to be infected. Most test-positive persons will go home and be given instructions as described in Storyboard A.
RNA tests (or potentially antigen tests) must provide: 1) very high sensitivity (>99%), 2) acceptable specificity, 3) reasonable throughput based upon the number of persons seeking testing per work shift, and 4) cost efficiency. Even though cost efficiency is not a major driver today (September 2020), in an endemic setting it will become essential. The fact that most RNA tests performed to date are negative suggests that triage of some form is necessary to effectively use testing capacity. Even at the height of infections in New York only about 25% of RNA tests were positive. In many places in the US fewer than 5% are positive. We recently spoke to a laboratorian colleague in a very low prevalence area who has found only 0.2% of tests positive after thousands of outpatient samples were tested.
The tests designed for the SARS-CoV-2 diagnosis (Use Case 5) will likely be used for other Use Cases including surveillance (Use Case 8), and environmental testing (Use Case 9).
The Storyboards are meant to be approximate descriptions of the overall testing ecosystem. They are organized as flow charts containing sites of activity, people involved (e.g., patients, medical practitioners and laboratorians) and pathways for tested individuals, healthcare professionals (or other testers in some cases), sample collection and transport, testing, result generation and information flow. They also show key decisions informed by the test results.
Steps in this Storyboard that are different from Storyboard A are shown with a thick blue outline.
There are three types of “journeys” in the Storyboards: 1) the tested individual’s (usually patient) journey which are shown using green arrows, 2) point-of-care or point-of-use activities which are shown with purple arrows (e.g., sample collection, sometimes testing) and 3) the sample and data journey through a laboratory, which is shown with blue arrows.
The letters that label each step are not meant to indicate an order for the steps, they are simply there to facilitate discussion about the storyboard. Optional steps have a dashed outline, and examples of possible variations in a step are labeled with the same letter followed by differentiating numbers e.g., B1, B2 and B3)
There are a number of clocks and calendars pictured near specific steps to indicate time-consuming steps and those that could vary in total time depending upon the workflow efficiency of the healthcare site and the characteristics of the testing platform (e.g., batch analysis, time to results).